|
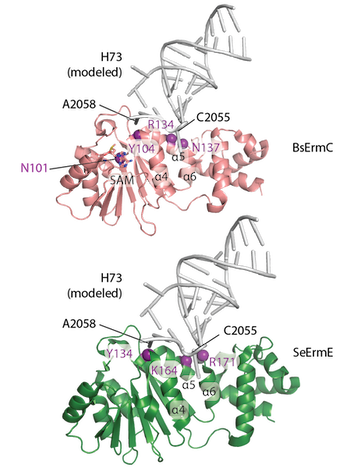
Erythromycin resistance methyltransferase (Erm) enzymes are a clinically important mechanism of multi-drug antibiotic resistance rendering bacteria resistant to macrolide, lincosamide and ketolide antibiotics. Whether or not all Erm enzymes bind to their rRNA substrate in a similar manner is an outstanding question. A new publication from the Dunkle group titled "Shared requirements for key residues in the antibiotic resistance enzymes ErmC and ErmE suggest a common mode of RNA recognition" appeared in the December 18 edition of the Journal of Biological Chemistry. The article demonstrates that similar conserved residues in ErmC or ErmE are required for function by deploying in vivo and in vitro assays on a collection of site-directed mutants. Also a structural model for how Erm enzymes bind rRNA is presented.
Comments are closed.
|
|
|
Accessibility | Equal Opportunity | UA Disclaimer | Site Disclaimer | Privacy | Copyright © 2020
The University of Alabama | Tuscaloosa, AL 35487 | (205) 348-6010 Website provided by the Center for Instructional Technology, Office of Information Technology |

 RSS Feed
RSS Feed
